It was reported yesterday that Pfizer will stop doing research and development in heart disease, anemia and osteoporosis to concentrate in other areas such as cancer, diabetes, and immunology/inflammatory diseases.
This is interesting since Pfizer has (and had) a large number of products in heart disease, including Lipitor, and pharmaceutical companies have typically continued to do research in areas where they have had products because they have established sales people who are knowledgeable about the disease area and have relationships with clinicians in those areas. The countervailing force is that many effective medicines to treat heart conditions (like high blood pressure and high cholesterol) are available in generic forms and thus the value bar (benefit/cost ratio) that new medicines must reach to be competitive is much higher than when they competing against other non-generic medicines.
However, one common and very expensive heart condition where better medical treatments are needed is heart failure – often called congestive heart failure (CHF). According the the National Heart Lung and Blood Institute, 5 million Americans have heart failure, and 300,000 die from it each year. The costs for these patients are very significant: Total costs for treating heart failure in the US are estimated to be $34.8 billion in 2008, and Medicare spent $4.7 billion for hospitalizations related to CHF in 2006.
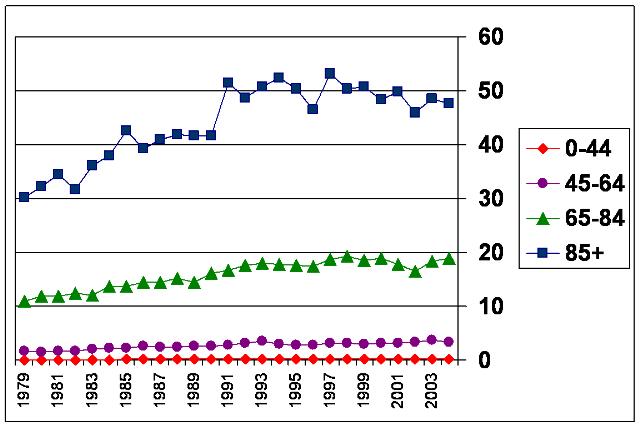
The chart below from the Centers for Disease Control and Prevention shows the rate of hospitalization (per 1000 people) for heart failure in the US by age group over the years 1979-2004 – clearly a growing problem.
Hospitalization Rate (per 1000 people) by Age Group for Heart Failure
1979-2004
So what does this mean for better treatments for CHF? According to the pharmaceutical industry’s web site database, there are currently 35 therapies in clinical trials for heart failure or congestive heart failure – including 3 based upon stem cell therapies, 1 based upon cell transplantation, and 1 using gene therapy. (This compares to the 105 therapies in development for cancer, and 81 for pain.) So I guess there will continue to be new treatments developed for heart failure, just probably not by Pfizer. But, recognizing that there are lots of medical problems and limited resources need to be prioritized, will this be OK for current and future patients with heart failure?
Companies allocate and prioritize research and development resources according to three fundamental factors:
- Unmet Medical Need
- Scientific Opportunities and Discoveries
- Market Potential
It is this last one that apparently Pfizer has decided has decreased, so they will be putting their resources into other areas where the combination of all three factors looks more appealing. As long as all the research-based biopharma companies don’t make those decisions in the same direction (i.e. into and out of the same diseases), then research resources will likely be allocated in a reasonable way to meet societal needs. In that way the needs of people with heart failure will be balanced against those with cancer, chronic pain, diabetes, neurological diseases and immune dysfunctions – which is what society and patients really should want, since people often have multiple diseases or medical problems, so they should want new and better treatments for all of them.
Addendum: The memo from Pfizer’s R&D leadership about their strategic realignment has been posted by Forbes – click here.

I lately came across your blog and have been reading along. I thought I would leave my first remark. I don’t know what to say except that I have enjoyed reading. Respectable blog. I will keep visiting this blog very often.